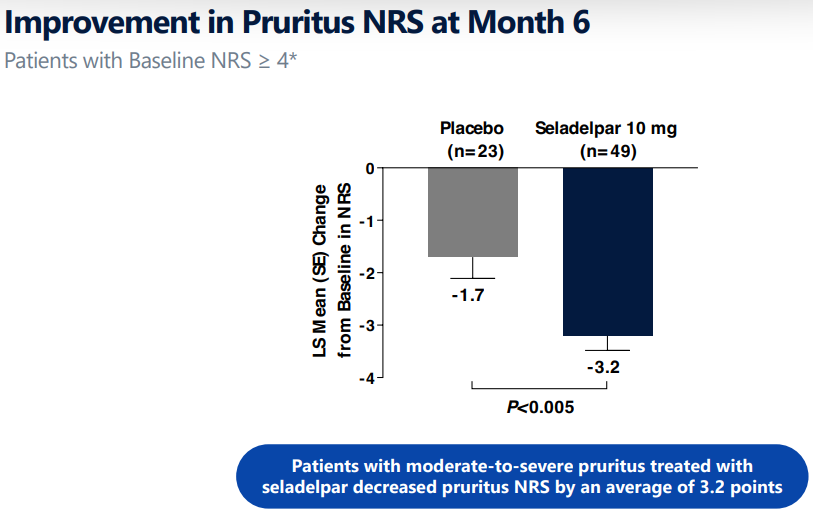
seladelparは痒みの有意な低下ももたらしました。痒みの主観評価NRSがもとは4以上(中等~重度の痒み)のseladelpar治療患者49人のNRSが半年後には3.2点下がり、プラセボ群の1.7低下を2倍ほど上回りました。NRSは下限が0、上限が10の検査値で、点数が大きいほどより重度です。NRSの0点はまったく痒くないことを意味し、10点は最悪に痒い(worst imaginable itching)ことを意味します。
今回の試験結果を頼りに米国FDA、英国MHRA、欧州医薬品庁(EMA)に同剤を承認申請できるとCymaBay社は言っています。
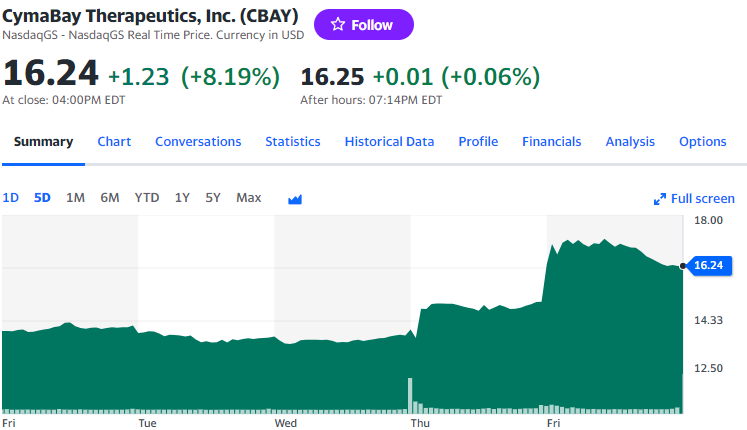
今回の木曜日朝の発表を受けてCymaBay社の株価は果たして上昇しています。水曜日の終値は14ドルほどだったのが金曜日には一時17ドルを超え、16ドル台で取引を終えています。
PBCはもっぱら女性が罹る肝臓の慢性炎症疾患です。稀な病気であり、米国の女性の千人あたり1人、数にして約13万人がPBCを患います。
Seladelpar RESPONSE Phase 3 PBC Study Topline Results September 7 | 2023 /
CymaBay
https://content.equisolve.net/cymabay/media/5877e69ece766c47377ee441f22df752.pdf
CymaBay jumps on data for primary biliary cholangitis therapy seladelpar /
FirstWord https://firstwordpharma.com/story/5778529
CymaBay’s Seladelpar Achieves High Statistical Significance for the Primary
and Key Secondary Endpoints in the Phase 3 RESPONSE Trial in Primary
Biliary Cholangitis / PRNewswire
NEWARK, Calif., Sept. 7, 2023 /PRNewswire/ — CymaBay Therapeutics, Inc.
(NASDAQ: CBAY), a biopharmaceutical company focused on innovative therapies
for patients with liver and other chronic diseases, today announced
positive topline results from its Phase 3 pivotal RESPONSE study. The study
evaluated the safety and efficacy of seladelpar, a potent, selective,
orally active delpar or PPARδ agonist, in development for the treatment of
adult patients with primary biliary cholangitis (PBC). The registration
trial achieved the primary and all key secondary endpoints and supports
advancement to regulatory discussions and filing for regulatory approval
with the U.S. Food and Drug Administration (FDA), the Medicines and
Healthcare products Regulatory Agency (MHRA), and the European Medicines
Agency (EMA).
A total of 61.7% of patients on seladelpar 10 mg (n=128) met the primary
composite endpoint related to serum alkaline phosphatase and bilirubin at
12 months versus 20.0% on placebo (n=65; p<0.0001). The anti-cholestatic
effect of seladelpar was supported by the normalization of alkaline
phosphatase at 12 months (key secondary endpoint) in 25.0% of patients on
seladelpar vs. zero on placebo (p<0.0001). The least-squares mean percent
reduction in alkaline phosphatase at 12 months was 42.4% in the seladelpar
group vs. 4.3% in the placebo group (p<0.0001). Seladelpar treatment compared to placebo also demonstrated a statistically
significant reduction in pruritus, or itch, (key secondary endpoint) after
6 months of treatment. Seladelpar-treated patients with a baseline
Numerical Rating Scale (NRS) ≥4 (moderate to severe pruritus) had a
least-square mean reduction of 3.2 points in pruritus NRS (n=49) compared
to 1.7 points for patients in the placebo group (n=23; p<0.005). Overall, safety was comparable between placebo and seladelpar groups and
was consistent with previous studies. Treatment-emergent adverse events,
serious adverse events, and patient discontinuations were generally
balanced across the treatment and placebo arms. There were no
treatment-related serious adverse events in the study. Seladelpar’s
tolerability profile appeared favorable and consistent with previous
studies.
“The topline results seen in the RESPONSE trial are exciting for
highlighting the potential for an efficacious and safe new therapy that not
only achieves the composite improvements in liver tests, but for a
significant proportion of patients, normalizes these measures. Further, the
results support that seladelpar reduced itch, a particularly challenging
symptom that continues to negatively impact quality of life for many PBC
patients,” said Gideon Hirschfield, M.D., Lily and Terry Horner Chair in
Autoimmune Liver Disease Research, Toronto Centre for Liver Disease. “While
existing first and second-line therapies have helped patients living with
PBC, this is the first potential therapy to show promise in both
significantly improving markers associated with risk of disease progression
while also significantly reducing itch.”
“The results from RESPONSE support our conviction that seladelpar has the
potential to advance patient care by improving measures of disease activity
and reducing symptom burden. They are consistent with previous findings in
what we believe has been an exceptionally robust development program in
PBC. We believe that the delpar mechanism is unique with its ability to
normalize markers of cholestasis coupled with reductions in pruritus,” said
Sujal Shah, President and CEO of CymaBay. “Many patients with PBC suffer
from incessant itching while knowing that their disease can progress to the
point where a liver transplant could become their only option. These
results represent an important step toward potentially changing the
treatment paradigm for patients living with PBC. We deeply appreciate the
participation of patients across all of our studies in PBC and the support
we have received from investigators, their teams and our many partners
involved in advancing the development of seladelpar through this
significant milestone.”
Additional analyses of RESPONSE are ongoing, and the company looks forward
to sharing additional data in an upcoming medical meeting.
RESPONSE was a double-blind, placebo-controlled, global study of one-year
duration that randomized 193 PBC patients in a 2:1 ratio to seladelpar 10
mg or placebo, once daily. Eligible patients had an inadequate response or
intolerance to ursodeoxycholic acid (UDCA) with serum alkaline phosphatase
(ALP) ≥ 1.67× the upper limit of normal (ULN) after at least 12 months of
treatment. The primary outcome measure was the responder rate defined as a
patient who achieved an ALP level < 1.67× ULN with ≥ 15% decrease in ALP,
and total bilirubin (TB) ≤ 1.0× ULN after 52 weeks.
Secondary outcome measures were the proportion of patients with ALP ≤ 1.0×
ULN at 12 months and the change from baseline at 6 months in the
patient-reported level of pruritus as assessed by the NRS in those patients
with baseline NRS ≥4. The NRS is a scale of 0 (no itching) to 10 (worst
imaginable itching). At baseline, mean ALP levels were 314.3 U/L and TB
0.76 mg/dL and the mean baseline NRS was 6.3 in those patients evaluated
for the pre-specified pruritus endpoint. The baseline characteristics were
balanced between the two groups and representative of a high-risk PBC
patient population with a high level of symptom burden.
Conference Call
CymaBay will host a conference call today, Thursday, September 7 at 8:00
a.m. ET to discuss the topline results from this study. To access the live
conference call, please dial 877-407-0784 from the U.S. and Canada, or
201-689-8560 internationally, Conference ID# 13741034. To access the live
and archived webcast of the conference call, go to the Investors section of
the CymaBay website at ir.cymabay.com/events. A slide presentation
to be referenced on the conference call will be available in the Investors
section of the CymaBay website shortly before the call.
About PBC
PBC is a rare, chronic inflammatory liver disease primarily affecting women
(1 in 1,000 women over the age of 40 or about 130,000 total people in the
US). PBC is characterized by impaired bile flow (known as cholestasis) and
the accumulation of toxic bile acids in the liver, leading to inflammation
and destruction of the bile ducts within the liver and causing increased
levels of ALP and total bilirubin. The most common early symptoms of PBC
are pruritis (itching) and fatigue, which can be debilitating for some
patients. Progression of PBC is associated with an increased risk of
liver-related mortality.
About Seladelpar
Seladelpar, an investigational treatment for people with PBC, is a
first-in-class oral, selective peroxisome proliferator-activated receptor
(PPAR) delta agonist, or delpar, shown to regulate critical metabolic and
liver disease pathways in indications with high unmet medical need.
Preclinical and clinical data support its ability to regulate genes
involved in bile acid synthesis, inflammation, fibrosis and lipid
metabolism, storage, and transport.
About CymaBay
CymaBay Therapeutics, Inc. is a clinical-stage biopharmaceutical company
focused on improving the lives of people with liver and other chronic
diseases that have high unmet medical need through a pipeline of innovative
therapies. Our deep understanding of the underlying mechanisms of liver
inflammation and fibrosis, and the unique targets that play a role in their
progression, have helped us receive breakthrough therapy designation (U.S.
Food and Drug Administration), Priority Medicines status (European
Medicines Agency) and orphan drug status (U.S. and Europe) for seladelpar,
a first-in-class investigational treatment for people with PBC. Our
evidence-based decision-making and commitment to the highest quality
standards reflect our relentless dedication to the people, families, and
communities we serve. To learn more, visit www.cymabay.com and follow us on
X (formerly Twitter) and LinkedIn.

コメント