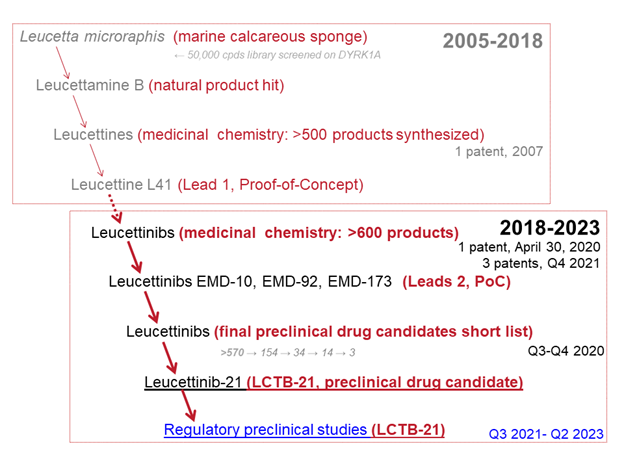
フランスの製薬会社Perha Pharmaceuticalsがダウン症候群やアルツハイマー病の認知障害の治療として開発しているDYRK1A阻害剤Leucettinib-21 (LCTB-21)が生み出されるまでの過程。Leucettinib-21は海の海綿動物の成分を起源とし、2026年末までに臨床試験段階に進む見込み。
Down Syndrome and Alzheimer’s Disease share a common therapeutic target involved in cognitive disorders : the DYRK1A protein kinase. Leucettines and Leucettinibs, the low molecular weight DYRK1A inhibitors optimized by Perha Pharmaceuticals, are derived from the natural product Leucettamine B, extracted from the calcareous sponge Leucetta microraphis.
The Leucettine family was first patented in 2007 as DYRK1A kinase inhibitors. The second-generation inhibitors, Leucettinibs, were patented in April 2020 and October 2021.
These DYRK1A inhibitors correct cognitive impairments observed in three mouse models of Down Syndrome and three rodent models of Alzheimer’s Disease. The preclinical drug candidate, Leucettinib-21, has been selected from over 600 synthesized Leucettinibs following a medicinal chemistry plan driven by efficacy on molecular, cellular and animal models, and a go/no go decision tree based on pharmacological criteria and drug safety.

コメント