CXCR4/CXCL12伝達亢進のせいで白血球が骨髄から出難くなって巡りが乏しくなることで生じる親譲りの稀な免疫不全疾患・いぼ、低ガンマグロブリン血症、感染症、骨髄性細胞貯留(WHIM)症候群を治療するCXCR4遮断経口薬mavorixafor(マボリキサフォル)をX4 Pharmaceuticals社が米国FDAに承認申請しました。
去年2022年の暮れにX4社はWHIM症候群への同剤の第3相試験成功を報告しています。また、今春4月にはその結果詳細をClinical Immunology Society (CIS) 年次総会で発表しています。
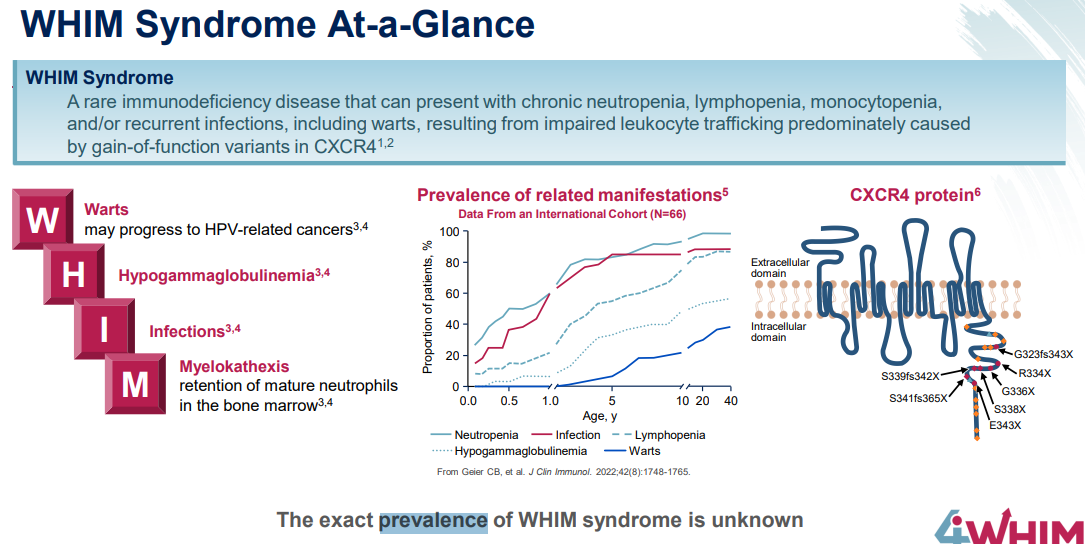
WHIM症候群の有病率はよくわかっていません。
X4 Pharmaceuticals Announces Submission of New Drug Application (NDA) to
U.S. FDA for Mavorixafor in WHIM Syndrome / GLOBE NEWSWIRE
https://www.globenewswire.com/news-release/2023/09/05/2737277/0/en/X4-Pharmaceuticals-Announces-Submission-of-New-Drug-Application-NDA-to-U-S-FDA-for-Mavorixafor-in-WHIM-Syndrome.html
Submission supported by positive results from global, pivotal 4WHIM Phase 3 clinical trial
BOSTON, Sept. 05, 2023 (GLOBE NEWSWIRE) — X4 Pharmaceuticals (Nasdaq: XFOR), a company driven to improve the lives of people with rare diseases of the immune system, today announced the submission of a New Drug Application (NDA) to the United States Food and Drug Administration (FDA) for the approval of once-daily, oral mavorixafor to treat individuals aged 12 and older with WHIM (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) syndrome, a rare, primary immunodeficiency.
“The submission of our first NDA is a significant milestone in X4’s journey to transform the care of those living with rare immunodeficiencies,” said Paula Ragan, Ph.D., President and Chief Executive Officer of X4 Pharmaceuticals. “We’re excited that this submission moves us one step closer to introducing what could be the first approved product in the U.S. for those with WHIM syndrome. We also continue to advance our clinical program evaluating mavorixafor in people with chronic neutropenic disorders.”
The FDA takes 60 days to determine whether an NDA is sufficiently complete prior to accepting it for filing. X4 has requested priority review for the application which, if granted, would provide a target FDA review period of six months from the application acceptance for filing date.
The NDA submission is supported by the results of the global, pivotal, 4WHIM Phase 3 clinical trial of once-daily, oral mavorixafor in individuals with WHIM syndrome. The 4WHIM trial met its primary endpoint and key secondary endpoint and was generally well tolerated in the trial, with no treatment-related serious adverse events reported and no discontinuations for safety events. The 4WHIM data also revealed that mavorixafor treatment resulted in reductions in the rate, severity, and duration of infections in trial participants versus placebo. These and additional 4WHIM Phase 3 data were published in oral presentations at the annual meetings of both the Clinical Immunology Society (CIS) and European Hematology Association (EHA).
About Mavorixafor and WHIM Syndrome
WHIM syndrome is a rare, inherited, combined immunodeficiency disease caused by reduced mobilization and trafficking of white blood cells from the bone marrow due to over-signaling of the CXCR4/CXCL12 pathway. WHIM syndrome is named for its four common clinical findings: Warts, Hypogammaglobulinemia, Infections, and Myelokathexis, although not all patients experience all symptoms, and not all symptoms are required for a diagnosis. People with WHIM syndrome characteristically have very low blood levels of neutrophils (neutropenia) and lymphocytes (lymphopenia), and as a result, experience frequent, recurrent infections with a high risk of lung disease, refractory warts from underlying human papillomavirus (HPV) infection, limited antibody production due to low levels of immunoglobulin, and an increased risk of developing certain types of cancer.
Mavorixafor is an investigational small-molecule antagonist of CXCR4 being developed as a once-daily oral therapy for WHIM syndrome. For the WHIM syndrome indication, mavorixafor has been granted Breakthrough Therapy Designation, Fast Track Designation, and Rare Pediatric Designation in the U.S., and Orphan Drug Status in both the U.S. and European Union.
About the 4WHIM Phase 3 Clinical Trial
The 4WHIM Phase 3 clinical trial was a global, randomized, double-blind, placebo-controlled, multicenter study designed to evaluate the efficacy and safety of oral, once-daily mavorixafor in people with genetically confirmed WHIM syndrome. The trial enrolled 31 participants aged 12 and older who received either mavorixafor (n=14) or placebo (n=17) orally once daily for 52 weeks. An open-label extension phase of the clinical trial is ongoing (NCT03995108).


コメント